Abstract
Background: For proliferation and survival, chronic lymphocytic leukemia (CLL) cells depend on interactions with cells and soluble factors present in the tumor microenvironment (TME). These interactions also increase expression of B-cell leukemia/lymphoma-2 (Bcl-2) proteins, including Bcl-XL, Mcl-1 and Bfl-1, thereby reducing drug sensitivity.
In the VISION HOVON141 clinical trial, patients with relapsed or refractory CLL are treated with 2 cycles of Bruton tyrosine kinase inhibitor ibrutinib (IBR) lead-in followed by 13 cycles of IBR + Bcl-2 inhibitor venetoclax (VEN) combination before randomization. The combination of IBR + VEN may have synergistic anti-tumor effects, since IBR forces CLL cells from lymph node (LN) to the blood (PB) where they become fully dependent on Bcl-2, and succumb to VEN.
To support a mechanistic basis for this premise, we investigated changes in expression of Bcl-2 proteins, effects of TME-mimicking signals, and venetoclax sensitivity before/after 2 months IBR treatment.
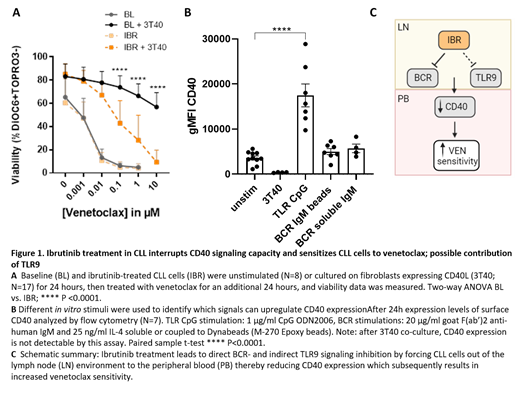
Results: At baseline, lymph node emigrant cells (CXCR4dim/CD5high) have higher Bfl-1 expression, in addition to earlier reported Bcl-XL and Mcl-1 expression than cells immigrating back to the lymph node (CXCR4high/CD5dim)(Haselager et al., 2020). After 2 months of IBR treatment a clear reduction in all four pro-survival proteins was observed (N=17 p<0.001). Despite these changes, VEN sensitivity was not different in unstimulated PB CLL cells obtained at baseline versus at 2 months of IBR treatment (N=8; IC50=0.001µM). In contrast, CD40 stimulated CLL cells obtained at baseline are fully resistant to VEN (IC50>10µM), but unexectedly retained partial sensitivity to venetoclax after IBR treatment (IC50=0.05 µM, p-value <0.0001; Figure 1A). This suggested reduced CD40 signalling capacity and was accompanied by reduced ability to upregulate Bcl-XL, Mcl-1, and Bfl-1 expression. Indeed, cell surface expression, as well as level of CD40 activation as measured by CD95 induction, was clearly reduced after 2 months IBR. Importantly, these effects occurred in vivo, as IBR did not directly affect CD40 signaling in vitro.
These data imply that under IBR treatment, when cells cannot (re-)enter LN sites, a factor is lacking that maintains or induces CD40 expression. In search of stimuli that can augment CD40 signaling capacity, it was found that BCR stimulation had no effect on CD40 expression. In contrast, TLR9 stimulation via CpG led to increased CD40 expression in CLL cells (N=7, p-value <0.0001)(Figure 1B). These findings align well with recent data which indicate a role for TLR9 signalling in vivo by unmethylated mitochondrial DNA in CLL (Kennedy et al., 2021). Taken together, these data provide a mechanistic explanation, by which a triad of signaling pathways not only involving BCR but also CD40 and TLR9 is interrupted by IBR (Figure 1C).
Discussion/conclusion: IBR treatment broadly affects Bcl-2 family protein expression and CD40 signaling in vivo. The combined data indicate a novel aspect of IBR efficacy, namely its capacity to interrupt TLR9-induced CD40 upregulation, which normally primes CLL cells in the LN environment for drug resistance. This also suggests that drugs that inhibit TLR9 signaling may synergize with IBR.
References
Haselager, M. V., et al (2020). Changes in Bcl-2 members after ibrutinib or venetoclax uncover functional hierarchy in determining resistance to venetoclax in CLL. Blood, 136(25), 2918
Kennedy, E., et al (2021). TLR9 expression in chronic lymphocytic leukemia identifies a promigratory subpopulation and novel therapeutic target. Blood, 137(22), 3064
Levin: Roche, Janssen, Abbvie: Other: Travel Expenses, Ad-Board. Westerweel: Novartis: Research Funding; Incyte: Consultancy; BMS / Celgene: Consultancy; Pfizer: Consultancy. Niemann: CSL Behring, Genmab, Takeda, Octapharma: Consultancy; Novo Nordisk Foundation: Research Funding; Abbvie, AstraZeneca, Janssen: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal